Throughout the early twentieth century the newly developed Haber–Bosch course of reworked meals manufacturing. Reliable entry to ammonia-based fertilisers was an unlimited improve to agriculture and helped to keep up the planet’s shortly rising inhabitants.
‘When Haber and Bosch discovered straightforward strategies to make ammonia artificially, that triggered a complete chemical revolution – it was the very first chemical course of used at that scale,’ explains Laura Torrente-Murciano, an expert in response engineering and catalysis based on the Faculty of Cambridge, UK. ‘It was created to be used as explosives, primarily, nonetheless indirectly, it is used proper now for fertilisers. And as a result of this reality, it sustains the world … spherical 50% of the world inhabitants are fed by ammonia made in chemical reactors.’
Nevertheless the Haber–Bosch course of hasn’t modified all that loads since its discovery better than 100 years prior to now. The tactic makes use of iron or ruthenium catalysts to react hydrogen and nitrogen collectively under extreme circumstances. Temperatures can attain 600°C, with pressures raised to over 200 situations that of the Earth’s atmosphere.
Ammonia goes to play a key perform throughout the decarbonisation of the society
Presently, massive Haber–Bosch reactors produce spherical 180 million tonnes of ammonia yearly, using up better than 1% of the general vitality produced worldwide. The tactic can be intertwined with fossil gasoline extraction – the hydrogen feedstocks are made by steam reforming coal and pure gasoline – and is straight accountable for 450 million tonnes of carbon dioxide emissions yearly.
With the urgent wish to chop society’s carbon emissions, Haber–Bosch is prolonged overdue a makeover. Many researchers now take into account that sustainably produced ‘inexperienced’ ammonia would not solely reduce the environmental have an effect on of fertilisers, nonetheless could also be used as a transparent vitality supplier.
‘For those who take into account the molecule itself, it is not that completely totally different to the methane molecule. So ammonia has one nitrogen, three hydrogens; methane, one carbon, 4 hydrogens. And that signifies that they retailer and launch vitality in a extremely associated technique,’ says Torrente-Murciano. ‘So I really feel ammonia goes to play a key perform throughout the decarbonisation of the society because of it might be utilized in a extremely associated technique that we use methane proper now.’
Engineering factors
Transforming ammonia manufacturing is susceptible to switch in two phases. The first entails adapting current manufacturing methods so that inexperienced hydrogen could be utilized as a feedstock, with renewable electrical vitality used to vitality the crops. Further into the long term, new methods that depend upon totally completely totally different chemistry might come on-line.

Whereas adapting current companies to utilize inexperienced hydrogen may sound easy, it is not pretty really easy because of current Haber–Bosch crops are solely deliberate throughout the steam reforming course of.
‘[The current Haber–Bosch process] it is designed spherical using fossil fuels as a result of the feedstock for hydrogen and as well as as a provide of vitality to run your entire course of. The difficulty is, everytime you’re trying to make inexperienced ammonia, that total integration disappears,’ says Torrente-Murciano. ‘In case you produce hydrogen one other method, it’s advisable think about strategies of working your compressors throughout the ammonia loop, the way in which you’re going to heat your reactors, the way in which you’re going to begin out up your methods and points like that – that requires a really new optimisation of the strategy.’
The very extreme pressures associated to Haber–Bosch help to maximise the amount of nitrogen and hydrgeon that is remodeled into ammonia in a single transfer, with out having to be fed once more into the reactor. In current companies, the compression methods are based on steam that may very well be a byproduct of the response that makes the hydrogen feedstock from fossil hydrocarbons. However when the strategy is to be based on inexperienced hydrogen, it would make sense to utilize far more energy-efficient electrical compressors.
Producing inexperienced hydrogen with renewable electrical vitality moreover brings additional challenges – chief amongst them being the problem of intermittency. Haber–Bosch companies are huge web sites which is perhaps designed to run 24 hours a day, seven days each week, requiring a gradual present of hydrogen to feed the reactors.
One reply is to assemble up a hydrogen buffer – a giant retailer of hydrogen that is bolstered when the photo voltaic is out, or the wind is blowing, and which the ammonia plant can draw upon always. Nevertheless this may occasionally solely help to deal with short-term vitality fluctuations and brings important additional costs.
‘The alternative reply is the occasion of novel ammonia synthesis utilized sciences which is perhaps able to shut down manufacturing and return as soon as extra counting on whether or not or not it is sunny or not,’ says Torrente-Murciano. ‘And that’s an unlimited change of mind-set regarding the chemical commerce … However after we’re ready to do that, it would seemingly be transformative.’
Torrente-Murciano and her colleagues have currently demonstrated a system by means of which ammonia is separated by absorption comparatively than condensation. This system operates at pressures as little as 30 bar and the manufacturing and separation of ammonia can occur in a single vessel, resulting in a far more versatile course of.
‘It is vitally necessary bear in mind that it’s a extraordinarily exothermic response, thus heat administration is an issue – particularly if we’re working the system in a dynamic technique,’ she gives. ‘We have now to be prepared for distributed manufacturing of ammonia to be aligned with the distributed nature of the renewable vitality – with potential crops orders of magnitude smaller than the current ones, the place dynamic operation may very well be additional manageable from a heat viewpoint.’
Lessons from nature
Previous merely modifying current Haber–Bosch processes, many researchers are attempting to design primarily other ways of developing ammonia. Nevertheless there’s a function why we’re nonetheless using a century-old course of. Nitrogen, which makes up nearly 80% of the air spherical us, is an exceptionally regular molecule. Nitrogen’s triple bond is the strongest homodiatomic bond acknowledged in chemistry, and breaking it is no straightforward job.
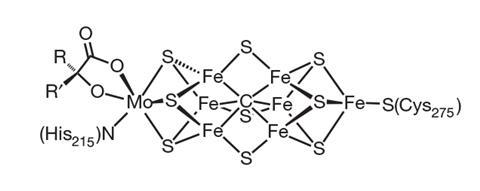
In nature the nitrogenase family of enzymes does the job. These enzymes are current in microbes that restore nitrogen into bioavailable ammonia. ‘You may speculate that throughout the evolution of the planet, flora really couldn’t have gotten very far if it hadn’t been for nitrogenase producing fixed nitrogen – because of the nitrogen content material materials of the current biosphere all comes from that enzyme,’ says Doug MacFarlane, whose group at Monash Faculty in Melbourne, Australia, is rising electrochemical means to supply ammonia.
MacFarlane explains that many researchers trying to find to develop new nitrogen-fixing processes are desperate to take lessons from how nature does it. There are three foremost programs of nitrogenase and all perform a core containing iron and sulfur atoms, nonetheless they’re distinguished from each other by the presence of molybdenum, vanadium or just iron.
‘For folk doing homogeneous and even heterogeneous catalysis, these look nearly like objects of a heterogeneous ground or they look like molecules you possibly can take into consideration to synthesise,’ says Serena DeBeer, whose lab on the Max Planck Institute for Chemical Vitality Conversion in Mülheim, Germany, makes use of an array of x-ray and spectroscopic methods to probe the nitrogenase enzymes’ train. ‘And so there’s loads of curiosity in understanding exactly how does this heterometal tune the reactivity? How does it permit a particular kind of reactivity?’
‘That said, all of these proteins are part of big multicomponent protein methods, they often’re moreover controlling the availability of electrons and protons,’ she gives. ‘And a number of what we’re trying to know is at each given step: the place are the electrons going? The place are the protons going? And the way in which does that change in these completely various kinds of the enzyme?’
‘They’ve dramatic variations in reactivity, that’s clear. They’ve intrinsically completely totally different catalytic energetic web site, that’s clear. But it surely absolutely seems to hold loads of intrinsic chemistry lessons designed by biology that we’re trying to tease out.’
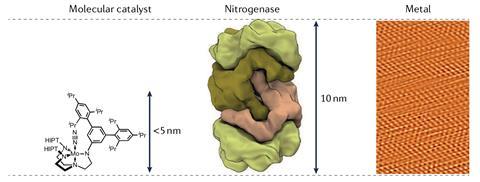
No matter a very long time of study into nitrogenase’s train, exactly how nitrogenases produce ammonia stays to be not completely understood. The precept function for that’s that nitrogenase itself is a uniquely refined enzyme.
‘Every step of one of the simplest ways, the crystallographers have been challenged, the spectroscopy has been challenged – part of it comes from the reality that it’s a tough protein to work with,’ says DeBeer. ‘Nevertheless part of it is merely even discovering out the character of such a complicated cofactor – it’s a cofactor with seven irons, one molybdenum, all open-shell, all spin-coupled … There isn’t a additional superior cofactor anyplace in biology.’
Even theoretical scientists wrestle to develop into accustomed to nitrogenase, and DeBeer notes that density sensible precept can’t completely describe the enzyme’s refined nature. ‘For practically all of the items we do, it has a level of complexity on the subject of the development, the magnetism, the mechanism that goes previous one thing now we now have a parallel to.’
Small molecule catalysts
Whereas there are quite a few question marks surrounding nitrogenase’s movement, the enzyme does provide pointers for these chemists trying to create totally different routes to ammonia. Key parts of the enzyme’s train are in one of the simplest ways it first binds the dinitrogen molecule after which delivers electrons and protons in a managed development to steer clear of merely making hydrogen. Avoiding this competing hydrogen-evolution response is a big downside for researchers working all through many different approaches to nitrogen low cost – from these rising small molecule catalysts to those engaged on electrochemical approaches.
In 2003, Dmitry Yandulov and Richard Schrock demonstrated the first occasion of a homogenous catalytic nitrogen low cost using a transition metal superior. This major molybdenum catalyst was partially impressed by the iron–molybdenum cofactor present in some nitrogenases, and extra molybdenum catalysts like these developed by Yoshiaki Nishibayashi on the Faculty of Tokyo, Japan, have since achieved nitrogen low cost prices approaching these of nitrogenases.
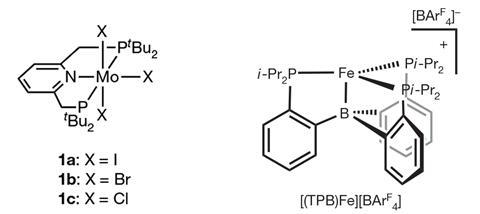
In gentle of rising proof that nitrogenase’s iron atoms may actually be additional important for binding nitrogen molecules, the group of Jonas Peters on the California Institute of Know-how developed the first iron-based catalysts which could be capable to altering nitrogen into ammonia. Nishibayashi’s group moreover developed iron catalysts using associated pincer ligands to those used with their earlier molybdenum methods. Catalysts based on totally different metals have moreover been demonstrated, nonetheless to this point solely the molybdenum ones work at room temperature, with others requiring cryogenic temperatures.
Independently of each other, Peters’ and Nishibayashi’s groups have very currently developed associated photocatalysts which could be capable to lowering nitrogen to ammonia.
‘The Haber–Bosch course of is a response pushed by the vitality of the reactants. For example, throughout the Haber–Bosch course of, hydrogen gasoline is the vitality provide,’ says Nishibayashi. ‘In distinction to conventional response methods along with the Haber–Bosch course of, ammonia synthesis using seen gentle is an ammonia synthesis method that makes use of vitality from exterior sources. We take into account that this achievement provides a chance to develop ammonia synthesis reactions using renewable vitality.’
The methods developed by every Peters and Nishibayashi’s groups use molybendum-based nitrogen low cost catalysts that features pincer ligands along with iridium photoredox catalysts. Every methods require sacrificial proton donors, and Nishibayashi notes that that’s one house that his group hope to reinforce.
‘Our future function is to develop an ammonia synthesis response using seen gentle vitality with water instead of dihydroacridine as a sacrificial lowering agent and hydrogen provide,’ he says. ‘We will even try to dramatically improve the quantum yield.’
Wiring the fixing
One of many essential promising approaches to artificial nitrogen low cost entails electrocatalysis. Some researchers have even used nitrogenase along with electrodes to carry out nitrogen low cost. Nonetheless, these methods are at a extremely early stage of development and are presently challenged by low prices, instability and poor effectivity.
Many alternative methods have been analysed using numerous sorts of electrodes. Nonetheless, this rising self-discipline has had points with reproducibility. Certainly one of many foremost factors for researchers investigating new methods is that until a catalyst reaches a positive diploma of train it’s fairly robust to tell whether or not or not any ammonia is produced electrocatalytically, or if it has derived from nitrogen-containing trace impurities which is perhaps present throughout the air and in solvents.
‘There’s an precise draw back in really proving [when] anybody’s carried out one thing notably spectacular on this self-discipline,’ says MacFarlane. ‘The difficulty is that the yield charge and the faradaic efficiencies are so low, that it’s pretty doable that [any ammonia] is coming from contaminants.’
To this point, the one electrocatalytic method for which there’s irrefutable proof of worthwhile nitrogen low cost entails splitting the dinitrogen bond using an electrode plated with metallic lithium. These methods often use an electrolyte original of an pure solvent containing a lithium salt and proton supplier – usually ethanol.
‘Mainly the necessary factor to enabling selective nitrogen low cost comparatively than binding protons and making molecular hydrogen is to constrain the entry to each electrons or protons,’ says electrochemist Ifan Stephens from Imperial Faculty London, UK. ‘That’s what happens in nitrogenase, and that’s what happens in molecular catalysts. And that’s what we anticipate is going on throughout the system we’re looking at, which is the lithium-mediated electrochemical system.’
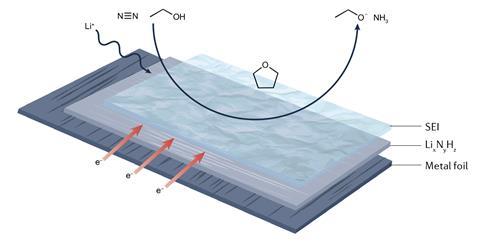
‘The sphere’s come a extraordinarily, really long way,’ notes Olivia Westhead, who works alongside Stephens at Imperial. ‘Nevertheless there’s nonetheless far more elementary understanding that we might like and far more optimisation to make it additional scalable and industrially associated.’
She notes that concentrations of salts, ethanol and water throughout the electrolyte can dramatically alter the effectivity of the lithium-mediated nitrogen low cost system. ‘It’s not going good industrially in case your system is so relying on this many parts,’ she says. ‘That’s one factor that should be appeared into – making it additional resilient to the small changes.’
Whereas early examples of lithium-mediated nitrogen low cost suffered from low effectivity and poor stability, newest analysis appear to have dramatically improved these metrics. A significant factor throughout the effectivity of lithium methods is the steady electrolyte interphase layer (SEI), which varieties on the ground of the unfavourable electrodes all through operation. The SEI improves the soundness of the electrode potential and as well as helps to constrain the number of protons that attain the electrode ground – serving to to forestall hydrogen evolution.
‘There’s been pretty numerous other ways of us have checked out addressing the [stability] draw back,’ says Westhead. ‘In our group, we seen that for many who merely enhance the salt focus, the unfavourable electrode potential stabilises and that’s since you improve your SEI – you make it far more inorganic and far more regular.’
‘Totally different groups have carried out it in one other method. [Researchers at] the Technical Faculty of Denmark found that for many who add a bit of little bit of oxygen, it stabilises your SEI layer as properly – so then you possibly can run it for ages,’ she gives. ‘As well as they develop a pulsing protocol the place for many who pulse your current the unfavourable electrode potential stays regular for for for much longer.’
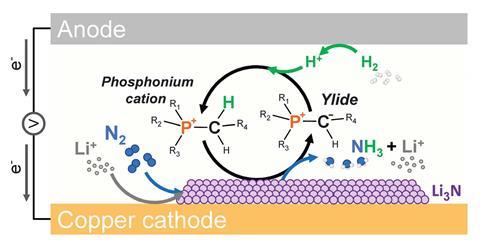
A variety of essentially the most dramatic enhancements have come from changes to the proton supplier and electrolytic salt. In 2021 MacFarlane’s group confirmed how utilizing a phosphonium proton shuttle, not solely helps to ship protons in a managed technique, however moreover elevated the ionic conductivity of the system.
‘The alternative issue that was pretty important was that the proton donation from the phosphonium and the reprotonation was all reversible – there is no sense by means of which this proton supplier could be utilized up,’ says MacFarlane.
Magic fluoride
On the an identical time, MacFarlane’s group was investigating the perform of the lithium salts. He explains that evaluation on lithium batteries has confirmed how important the perform of the salt could also be, nonetheless beforehand solely a small amount had been examined in ammonia low cost. Significantly, his group was serious about discovering out if fluorinated imide-based lithium salts may enhance the effectivity of the ammonia-producing cell.
‘It’s acknowledged throughout the self-discipline that these salts are very far more soluble in these ether solvents – in our case THF. And this allowed us to find a variety of concentrations vastly better than had been the classical work, which tends to be one molar or beneath, usually as little as 0.1 or 0.2 moles per litre – which is not loads of conductivity,’ he says. ‘Hastily in that work, we went all one of the simplest ways as a lot as about 4 moles per litre, which is loads of salt.’
By using this far more concentrated electrolyte, conductivity and current densities throughout the cells had been abruptly loads better. This drove up the train of the cell and achieved nearly 100% current to ammonia effectivity.
‘We realised that we had been attending to the aim of smart metrics. The US Division of Vitality laid out some metrics pretty a really very long time prior to now for what an electrochemical course of would look like for ammonia, they often said greater than 90% faradaic effectivity, [producing] spherical about one micro mole per sq. centimetre per second. So we had been getting upwards of a complete bunch of nanomoles, so we’re getting shut,’ notes MacFarlane.
Distributed reply
Primarily based totally on these developments, MacFarlane has organize a spin-out agency, Jupiter Ionics, that hopes to commercialise an ammonia-producing electrolyser. He envisages small devices which may run in every industrial greenhouse, producing merely ample fertiliser for specific particular person web sites. These new approaches to ammonia manufacturing have the potential to empower many communities which is perhaps presently totally reliant on distant companies and supply chains which is perhaps inclined to shocks from worldwide events.
‘Hastily fertilisers present could also be very loads an on-farm concept, and that’s why our first devices could also be pretty small,’ he says. ‘And now we now have had huge curiosity in that concept, which solely acquired additional substantial due to the Ukraine battle and the price hikes of gasoline and oil – because of most fertilisers come from pure gasoline – and supply constraints moreover.’
Fertiliser present could also be very loads an on-farm concept
This idea of smaller scale fertiliser producing fashions has gained traction in current occasions. For example, as a key part of its plan to cut back greenhouse gasoline emissions, Japanese meals agency AjinoMoto has partnered with Hideo Hosono, a provides scientist at Tokyo Institute of Know-how in Japan, to develop a low-temperature, low-pressure on-site ammonia manufacturing facility.
A additional distributed system of fertiliser manufacturing might also convey important monetary benefits to many rising nations. In a modern analysis, Torrente-Murciano confirmed how Sierra Leone might profit to the tune of $230 million (£180 million) over 30 years by using native hydropower to supply inexperienced ammonia comparatively than relying on imports. She believes that many alternative sub-Saharan nations may even see associated benefits.
‘There are a number of nations in Africa that don’t have entry to fertilisers, and that signifies that they put loads of emphasis on producing meals and your entire monetary system of these nations is based on agriculture merely to feed their inhabitants. We take into account that is what is limiting the precise development of the nation,’ she says. ‘You most likely have a look to what occurred in Europe many, a number of years prior to now, it wasn’t until you should have certainty that you just simply’re going to have the power to feed your inhabitants that people can start shifting jobs into additional industrialised ones – so we anticipate that agriculture and meals is on the idea of any developed society.’
Nonetheless, producing ammonia additional domestically and using it extensively as an vitality supplier brings additional challenges on the subject of safety. ‘The individuals who discover themselves coping with ammonia in the mean time are extraordinarily expert of us, and if we actually want to use ammonia as an vitality vector in a additional distributed technique, we’d wish to take into account very radical strategies of coping with ammonia – and that requires loads of know-how development,’ notes Torrente-Murciano.
Torrente-Murciano believes that fairly extra should be carried out to increase public consciousness of every the benefits and hazards associated to ammonia. ‘I don’t suppose that most people is aware that ammonia is way much less flammable than hydrogen by far [or] that you’d be capable to detect ammonia collectively together with your nostril at very low ranges,’ she says. ‘That is by itself very useful, because of you perceive when there is a leak, which couldn’t happen with hydrogen.’
‘We moreover wish to provide expert advice and truly get engaged with most of the people about ammonia – the merchandise and the bads. I really feel that we might like to concentrate to the challenges, comparatively than allowing of us to be scared as a result of lack of awareness,’ she gives. ‘That is one factor that your entire group should unify forces to deal with.’
Jamie Durrani is a senior science correspondent at Chemistry World