A model new technique to creating fluorine chemical substances may present a safer and additional sustainable totally different than present methods by circumventing the need to fabricate and take care of hazardous hydrogen fluoride. The strategy, developed by UK researchers, makes use of oxalic acid to amass a diffusion of fluorochemicals from the mineral fluorspar, which the workers says is scalable and works beneath delicate conditions in water at room temperature.

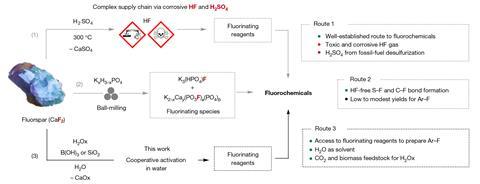
Fluorochemicals are traditionally derived from the mineral fluorspar, or calcium fluoride, and have wide-ranging functions, along with refrigeration, electrical vehicles, agrochemicals and prescribed drugs. At current, manufacturing of fluorine chemical substances requires reacting fluorspar with concentrated sulfuric acid at extreme temperatures – above 200°C. This produces hydrogen fluoride, which is used as a feedstock to amass a variety of fluorochemicals. Nonetheless, hydrogen fluoride is extraordinarily toxic and corrosive, posing in all probability deadly synthesis and coping with risks.
Now, Véronique Gouverneur’s lab on the School of Oxford, UK, has developed a safer course of to make a variety of commercially important fluorochemicals from fluorspar without having to make hydrogen fluoride or use large portions of fossil fuel-derived sulfuric acid.
‘The synthesis of fluorochemicals from fluorspar with no reliance on the superior present chain of hydrogen fluoride is probably going one of many best challenges in fluorine chemistry,’ says co-author Anirban Mondal. ‘We have now now overcome this downside with methods producing new and acknowledged fluorinating reagents.’
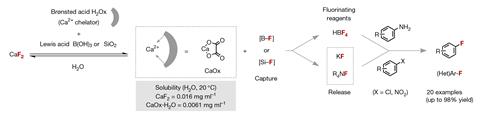
Getting down to find a fragile totally different to activate fluorspar – to primarily take away the calcium content material materials and entry the fluorine – the workers examined quite a few acids, landing on a course of that requires mixing three easy-to-handle solids: fluorspar, oxalic acid and each boric acid or silicon dioxide.
When the three solids have been mixed in water at room temperature the oxalic acid reacted with fluorspar to provide calcium oxalate, an insoluble salt. Within the meantime, the boric acid or silicon dioxide original sturdy boron–fluorine or silicon–fluorine bonds. The calcium oxalate was then eradicated just by filtration, abandoning an aqueous decision of each fluoroboric acid or hexafluorosilicic acid.
The workers then demonstrated that these choices may be remodeled using acknowledged fluorine chemistries to amass broadly used fluorochemicals, along with tetrafluoroboric acid, alkali metal fluorides, tetraalkylammonium fluorides and fluoro(hetero)arenes. ‘This work demonstrates unequivocally that the supply chain of hydrogen fluoride is simply not wanted for the manufacturing of fluorochemicals accessible upon nucleophilic fluorination,’ says Mondal.
Whereas moreover using a lot much less energy, the technique could also be further sustainable on account of oxalic acid may be comprised of carbon dioxide or biomass, whereas sulfuric acid required for the traditional technique is normally derived from fossil gasoline manufacturing. Mondal highlights {{that a}} downside for commercialisation is the current worth of oxalic acid, in distinction with sulfuric acid, nonetheless he stays optimistic. ‘Our protocol based mostly totally on oxalic acid aligns properly with the current challenges coping with the chemical commerce along with pathways to decarbonisation and defossilisation,’ he says.
‘We on a regular basis take into account hydrogen fluoride as a result of the entry stage to fluorine chemistry, whereas this work demonstrates that there is further that could be executed with calcium fluoride than beforehand thought,’ says Alan Brisdon, a fluorine chemist on the School of Manchester, UK. ‘Curiously, about 25 years prior to now I wrote an editorial for a fluorine know-how in-house journal that included speculation that “topics much like ‘activating’ calcium fluoride … will turn into further than merely tutorial curiosity”.’
Nonetheless, Brisdon components out that the size of present hydrogen fluoride manufacturing is giant and it is too early to say how this new technique might compete. ‘Its common utility will rely upon what totally different reagents or chemistry may be developed, nonetheless it’s a important step,’ he offers.
