Lasers might revolutionise nitrogen fixation, offering a model new method to synthesise ammonia beneath ambient circumstances. For the first time, researchers have used industrial carbon dioxide lasers to interrupt the nitrogen–nitrogen triple bond, offering a model new inexperienced totally different to the Haber–Bosch course of.
The worldwide group of researchers used lasers to remodel lithium oxide into metallic lithium, which spontaneously reacts with nitrogen in air to type lithium nitride. This salt is effectively hydrolysed into ammonia, breaking all current info by the use of yield.
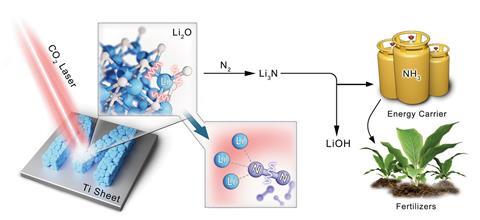
‘Now now we have launched a pioneering thought, [which] harnesses high-energy lasers to facilitate the conversion of various oxides into nitrides,’ says first author Huize Wang, from the Helmholtz Institute for Renewable Energy, in Germany. ‘Now now we have achieved an unprecedented yield […] beneath room temperature and atmospheric pressure circumstances, notable when compared with totally different methods,’ he gives. The yield is 2 orders of magnitude larger than totally different state-of-the-art choices, along with electrochemical and mechanochemical methods.
‘It’s a model new method for the manufacturing of inexperienced ammonia,’ says Victor Mougel, an expert throughout the electrochemical transformation of small molecules based at ETH Zurich, Switzerland. ‘[Alternative] methods are most likely further sustainable than the Haber–Bosch course of, which could possibly be very vitality intensive as a result of it operates at extreme temperature and pressure and […] contributes to carbon dioxide emissions.’ As the strategy works in ambient circumstances it ‘offers operational flexibility, along with the environmental benefits’. This course of might also allow ammonia to be produced the place it is wished, chopping the worth of transportation.
The group generated metallic lithium from lithium oxide, as a consequence of an infrared laser that offers adequate vitality to dissociate the lithium–oxygen bonds. When uncovered to air, metallic lithium spontaneously binds nitrogen breaking the nitrogen–nitrogen triple covalent bond and producing lithium nitride. ‘[We then] hydrolyse this laser-generated lithium nitride to amass ammonia gasoline and lithium hydroxide,’ explains Wang. Moreover, this technique offers the prospect for chemical biking. ‘A laser [can] induce the conversion of lithium hydroxide once more into lithium nitride, efficiently closing the lithium cycle,’ he gives. ‘This [is] moreover one different novel thought – the conversion from hydroxide to nitride.’
Ifan Stephens, an expert in electrochemistry and nitrogen fixation at Imperial College London, UK, stays to be sceptical. ‘I’m unsure [these] extreme fees is likely to be sustained for prolonged durations of time,’ he says. ‘Moreover, […] the reality that it is a batch course of, versus a gentle course of, would pose vital limitations to its viability.’ In distinction, electrochemical utilized sciences work repeatedly, which ‘offers a serious profit over the model new laser-induced method’, in response to Stephens. Furthermore, the vitality needs of the lasers might pose points for scaling-up ammonia synthesis. ‘Do you have to […] make ammonia on a small scale, as a fertiliser for distant locations, then the vitality effectivity turns into a lot much less obligatory,’ he gives.
‘Compared with electrochemistry, our method offers vital advantages [such as] desolvation and simplification,’ argues Wang. Plus, ‘the scale-up […] presents most likely a very powerful downside for all rising approaches for ammonia synthesis’. The researchers envision scaling up the strategy by distributing lithium oxide powder on a gridded flooring, then irradiating the arrays of response cells with the laser, sequentially. Furthermore, researchers have observed associated behaviour with totally different oxides, equal to magnesium, aluminium, zinc and calcium – although the yield is lower. ‘[It] might very effectively be on account of these totally different oxides are more durable to dissociate and hydrolyse,’ explains Stephens. Nonetheless, the reactivity of alkaline and alkaline earth metals within the path of nitrogen seems promising. ‘Our present work reveals that further appreciable metals, equal to magnesium and calcium can also dissociate nitrogen,’ he says.
