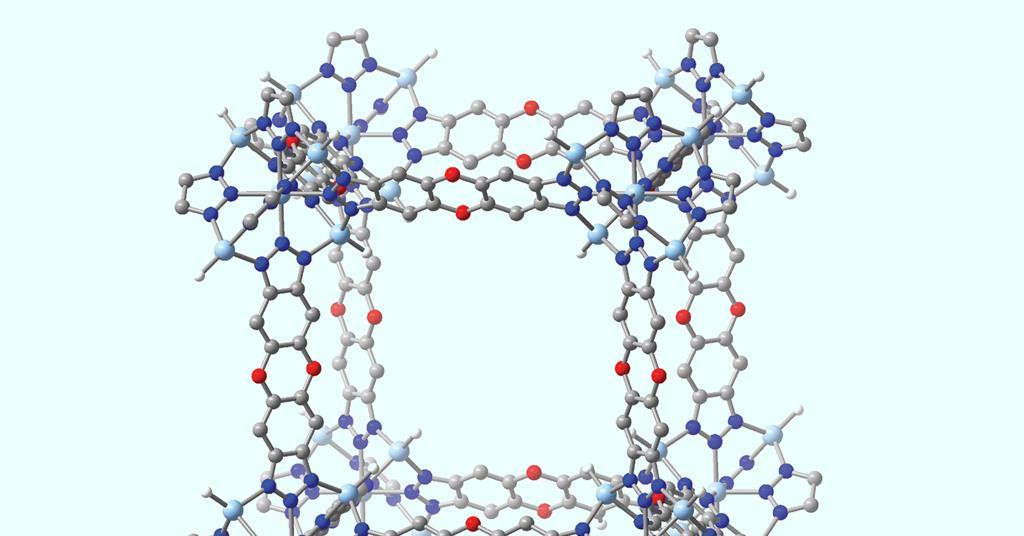
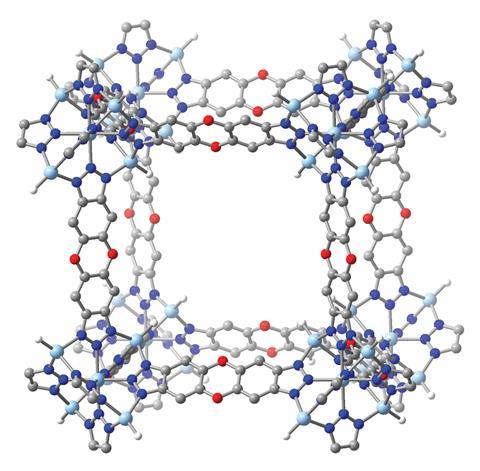
A model new metal–pure framework (MOF) development functionalised with terminal zinc hydride web sites can reversibly seize carbon dioxide at extreme temperatures.1 The material might enable easier, additional energetically atmosphere pleasant seize of carbon from the exhaust gases of many industrial reactions. In future, it may also lower the temperature wished for some key processes.
In all probability probably the most mature utilized sciences for capturing industrial carbon emissions are aqueous amines, nevertheless they solely work at temperatures spherical 60°C or lower, whereas the exhaust gases from steel furnaces and cement kilns are vented at over 200°C. ‘You’re going to grab the carbon dioxide at low temperature after which launch it at extreme temperature,’ says Jeffrey Prolonged at Faculty of California, Berkeley. ‘Temperature swings like which are sometimes inefficient.’
As a substitute, Prolonged’s group has been experimenting with amine functionalisation of MOFs, which, as porous cage constructions, are excellent for filtration. ‘Pure amines have various ranges of freedom, and when you seize carbon dioxide two of [the amines] come collectively to make an ammonium carbamate ion pair – that locks them into place,’ notes Prolonged. Which suggests there’s a giant entropy change associated to capturing carbon dioxide with pure amines that disfavours the response at extreme temperatures, he explains.
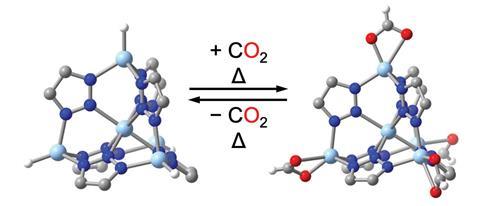
In 2014, Dirk Volkmer on the Faculty of Augsburg in Germany and colleagues confirmed that formates of a MOF known as MFU-4l functionalised with zinc hydride groups might launch carbon dioxide at low pressures.2 ‘What they didn’t realise is the response might presumably be reversible at extreme enough temperatures,’ says Prolonged.
Now, Prolonged and his colleagues have confirmed experimentally and computationally that, when uncovered to mixtures similar to flue gasoline from steel manufacturing, the material reversibly varieties metastable formates with the carbon dioxide at temperatures above 200°C. As zinc hydride has few ranges of freedom, the formation has little entropic penalty. When the carbon dioxide focus was lowered, the formate complexes broke down, releasing the carbon dioxide.
The researchers in the intervening time are investigating the potential of comparable MOFs to grab totally different gases at extreme temperatures. This would possibly paradoxically end in high-energy reactions requiring lower temperatures. ‘Numerous very high-energy reactions similar to the water–gasoline shift response that are run at 200 to 500°C are in equilibrium circumstances,’ Prolonged says. ‘One thought is to utilize a MOF … to actively seize carbon dioxide as a result of it’s produced throughout the response.’ Driving the equilibrium within the path of the required product might conceivably lower the temperature required. ‘For just a few of those methods, you may have the power to drop the response all the way in which right down to underneath 200°C after which use geothermal energy to start driving the response,’ he supplies.
‘There are many papers carbon dioxide seize nevertheless that’s the main one I’ve seen that works at these kinds of extreme temperatures,’ says Mircea Dinca at Massachusetts Institute of Experience throughout the US. ‘That’s positively the issue that distinguishes it from totally different works. The discovering could also be essential – and sudden I would say – first from a primary standpoint, nevertheless doubtlessly just about too.’