A model new organocatalyst might reduce the ability use of a vitally needed chemical course of – the chlor-alkali course of. Yadong Li from Tsinghua School in China, and his employees found that comparatively low value pure quinazoline-2,4-diones cut back the ability consumption of the tactic in distinction with pricey metallic catalysts. If such a catalyst had been adopted worldwide by the chlor-alkali commerce, it could save roughly 1.8–4.6% of the 150TWh it consumes yearly, Li’s employees finds. That may roughly equate to between three to 9 days of widespread day-after-day UK energy consumption. Completely different scientists say the catalyst’s stability nonetheless desires an entire lot of labor sooner than such energy monetary financial savings might probably be realised.
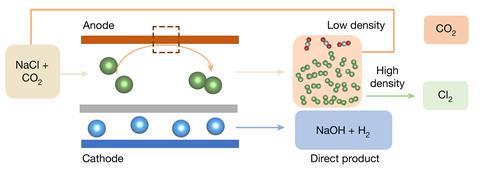
The chlor-alkali course of passes electrical vitality by way of a sodium chloride reply, producing chlorine and sodium hydroxide. The European Price has estimated that this course of underpins higher than half of the continent’s chemical commerce product sales, with 2 million jobs linked to its merchandise. The chlor-alkali course of’s significance is matched by its energy depth, as a result of it consumes spherical 4% of {the electrical} vitality the world produces.
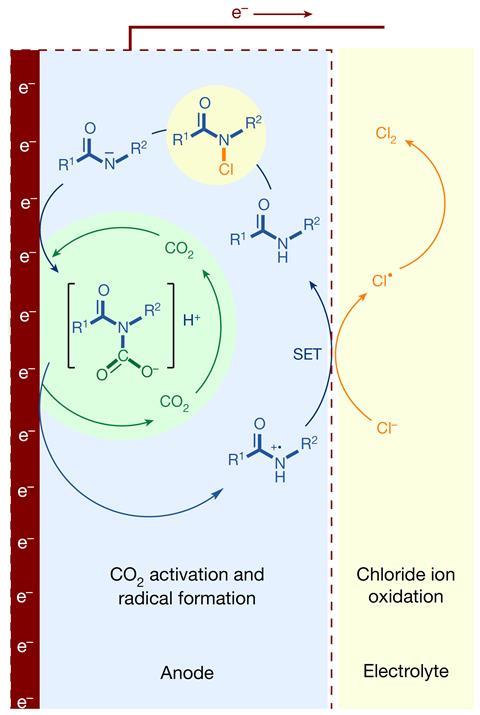
Traditionally, the chlor-alkali course of happens in membrane cells, reactors with two chambers, each containing an electrode. Electrical current passes by way of a concentrated salt reply inside the first chamber, which contains a dimensionally safe anode (DSA), releasing chlorine. The DSA is generally titanium coated with a conductive oxide of a noble metallic like ruthenium and likewise corrosion-resistant titanium dioxide. The other chamber separates water into hydrogen and hydroxide ions on the cathode. Sodium ions switch from the first chamber to the second and blend with hydroxide ions to make sodium hydroxide.
Harsh setting
Industrial efforts to make the chlor-alkali course of as a lot as 25% additional setting pleasant are already happening. This entails together with oxygen to provide additional hydroxide ions from plenty of the hydrogen gas that may in another case be launched. This response changes {the electrical} potential on the cathode, decreasing the potential distinction between electrodes. That lowers the membrane cell’s voltage, and subsequently its energy consumption. That’s the methodology that Li and his employees have now sought to further improve.
Li’s employees modified the DSA with pure molecules containing amide groups supported on an electrode product of titanium and carbon. As well as they bubble carbon dioxide by way of the cell, which reacts with the amide compounds to provide carbamic acid intermediates, enabling catalytic cycles that produce chlorine with good effectivity. One key downside with this new catalyst is that its train slowly decays with use, although the employees believes {that a} higher preparation approach may cease this.
Rolf Hempelmann from the School of Saarland calls the analysis ‘a pleasing piece of instructional work’ nonetheless doubts its industrial relevance. ‘Chlorine gas at oxidising electrochemical potential is the harshest chemical setting one can take into consideration,’ he says. One of the best ways that the catalyst system is in the meanwhile constructed cannot compete with the 10-year lifespan of those that are in the meanwhile used, he asserts.
‘Will in all probability be very troublesome to comprehend the required sturdiness over quite a lot of years,’ agrees Thomas Turek from the Technical School of Clausthal, who wrote a data and views article to accompany the model new analysis. Nevertheless he doesn’t contemplate that it is unimaginable. ‘The great price of the publication, in my view, is that for the first time an pure catalyst with these wonderful properties for chlorine evolution has been demonstrated,’ he gives. ‘It is a first step, and clearly further evaluation effort is required.’
